Don't Worry, AI Cannot Takeover the World, It will Run Out of Battery
The existential crisis of a Battery's Life, What's being done to solve it, and Characteristics of an Ideal Battery
Are you worried about AI? Do you think it’s going to takeover your job? Are you afraid your spouse might prefer your AI version over you? Have no fear! It is not the government, the big tech, or the academia, who you have to rely upon to save you (and possibly your marriage). Its the battery in your device (and its measly little life), that’s going to save the day. All hail the Lithium-ion!
Are you having difficulty taking me seriously? I completely understand. I don’t take me seriously either. But what I am saying does hold weight. Think about it.
Let’s say our buddy Elon develops the first ever in-house AI robot for doing all your home chores. You turn it on, and lo and behold, it lights up and listens to your every command. It scurries around, cleaning, cooking, and washing, and life is good.
But right when you really need it, instead of helping, it retreats back to its charging station to stay dormant for 3-4 hours (best-case) until it can walk again. Perhaps that notion doesn’t bother you very much. In fact, that’s what humans do. We retreat into our bedrooms so we can sleep and charge for a good 6-8 hours before we are functional again.
But isn’t that exactly the problem that we are trying to overcome using robots? Creating machines that, unlike us humans, don’t run out of battery? Worse yet, the performance of this robot is going to decline with every single recharge because that is how batteries are designed.
Okay, forget the future. What about the present? Why do we keep charging our phones, earbuds, and Fitbits without ever questioning what life could be like if we never had to charge our devices again? Imagine going to bed without worrying about your phone running out of charge. Imagine just using your earbuds all the time without having to ever plug them in.
The worst part of my day is when I see the yellow blinking light on my Google Earbuds indicating it is running out of charge. It has this uncanny way of happening right when I REALLY NEED my headphones. Also, I cannot count the number of times I had an inspiration to write in the nature, took my laptop and coffee, and rushed to the park near my house, only to open my laptop and realise I have 5% battery left.
Batteries Suck! Yes. They do. Even Lithium-ion. Should I stop complaining and just be grateful for what I have? No, that’s not how technology progresses. So in this article, we are going to question WHY we have to keep charging our devices, WHAT’S being done to solve it, and what makes an IDEAL BATTERY. Let’s get started.
The Life and Death of a Battery
First, let us begin with understanding how a battery works. If you are a battery making, robot creating, space-jumping, Ironman, feel free to skip this section. But for mere mortals such as myself, getting the basics right is necessary.
So let us deconstruct the battery by taking the most commonly used rechargeable battery today - Lithium-ion.
Stage 1: Birth
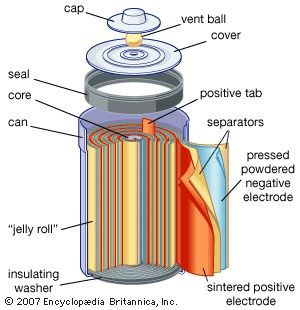
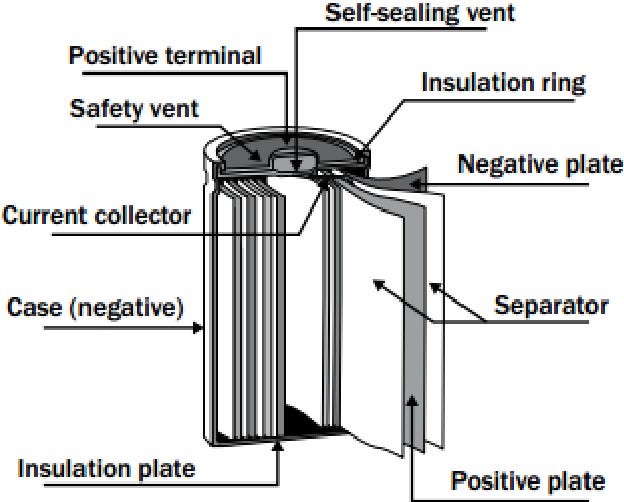
The lithium-ion battery's life commences in a fabrication facility where distinct components, including the anode, cathode, electrolyte, and separator, are meticulously assembled.
Anode: Typically made of graphite, the anode stores lithium ions during the charging process.
Cathode: Composed of lithium metal oxide, the cathode is the destination of the ions during discharge, releasing energy.
Electrolyte: A lithium salt in an organic solvent that facilitates the movement of lithium ions between the anode and cathode.
Separator: A porous material that prevents the anode and cathode from making direct contact, averting short circuits.
Stage 2: Adolescence
As the battery breathes life, an initial charge calibrates its internal chemistry. Lithium ions migrate from the cathode to the anode, nestling within the lattice of the graphite anode. You can see the process in Figure 1. This initial charge is crucial; it sets the tone for the battery’s performance and longevity.
Stage 3: Adulthood
The core of the battery’s life is characterised by repeated cycles of charging and discharging.
Charging: External power instigates the migration of lithium ions from the cathode to the anode. Electrons are simultaneously pushed into the anode externally and are barred from traversing through the battery, forcing them through the external circuit, providing power to the device.
Discharging: When the device is in use, electrons travel from the anode through the external circuit, powering the device. Inside the battery, lithium ions migrate back to the cathode through the electrolyte.
Stage 4: Mid-Life Crisis
Over time, the repeated cycles of charging and discharging, coupled with operational and environmental stresses, instigate degradation. And just like humans, the battery enters into a state of mid-life crisis. Fortunately, it doesn’t go get a tattoo or buy a motorcycle, instead it increases its internal resistance to the process of charging and discharging, thus leading to a steady loss in performance.
Capacity Fade: Each cycle results in a slight decline in the battery’s capacity due to undesirable chemical reactions, such as the formation of a solid electrolyte interface (SEI) on the anode.
Internal Resistance Increase: Impedance rises due to the SEI’s growth and other degradation mechanisms, reducing the efficiency of ion and electron flow.
Stage 5: Senescence
The battery’s capacity and efficiency to hold and deliver power erode. The device’s operational time between charges dwindles; the grace of peak performance fades into the echoing silence of diminished energy.
Stage 6: Death
Ultimately, the lithium-ion battery succumbs to the accumulated effects of internal chemical reactions and external operational factors.
Complete Capacity Loss: The battery can no longer hold a charge, rendering it incapable of powering a device.
Recycling or Disposal: In death, the battery either finds its way to recycling facilities to reclaim some of its components or faces disposal, marking the end of its journey.
And on that positive note, let us end this section and move on to the next.
Different Devices, Different Batteries
While Lithium-ion batteries are what most portable devices use these days, there are at least a couple others that I would like to mention.
Nickel-Cadmium (NiCd)
In the yesteryears of portable electronics, NiCd batteries were the favoured child. Robust and endowed with a commendable lifespan, they were, however, infamous for the ‘memory effect’—a phenomenon where the battery ‘forgets’ its full capacity if not regularly drained and recharged completely. The environmental footprint of cadmium, a toxic heavy metal, also casts a dark shadow on its legacy.
Nickel-Metal Hydride (NiMH)
Emerging as a successor to NiCd, NiMH batteries boast a superior energy density and are devoid of toxic constituents. While they grapple with the memory effect to a lesser degree, their lifespan doesn’t hold a candle to lithium-ion.
Why Lithium-ion is a Good Idea
Why did we choose Lithium-ion as the market standard for portable user electronics? The answer is primarily 3-pronged.
Structural Benefits
When juxtaposed with its predecessors, Li-ion batteries present an architectural finesse that is hard to ignore. Their lightweight design, paired with the ability to morph into diverse shapes, gives product designers the creative liberty to innovate. The absence of the memory effect, that plagued NiCd batteries, marks another structural advantage, freeing users from the rigorous charging cycles.
These batteries aren't just compact; they are engineered to be responsive, with a capability to release large bursts of energy on demand, a feature that is necessary in applications where rapid power deployment is critical.
The Lithium Advantage
Lithium is great for storing energy. It is lightweight with great electrochemical potential allowing it to store and release huge amounts of power on-demand.
Lithium's reactive nature is deftly harnessed in Li-ion batteries, allowing for swift electron movement and ensuring that your gadgets are not just powered, but empowered to deliver optimal performance.
Energy Density
So far, Li-ion is unrivalled in the market when it comes to energy density. These batteries pack an impressive amount of energy within their compact frames. This trait not only extends the lifespan of electronic devices between charges but also supports the energy-hungry functionalities in modern gadgets.
Just FYI, Energy density refers to the amount of energy stored per unit volume or mass in a system or material. There are two types of energy densities
Gravimetric Energy Density (Wh/kg):
It measures the amount of energy stored per unit mass (kilogram) of the battery.
This is crucial for applications like electric vehicles and drones, where the weight of the battery significantly impacts performance and efficiency.
Volumetric Energy Density (Wh/l):
It quantifies the energy stored per unit volume (litre) of the battery.
This is important for portable electronics like smartphones and laptops, where the size of the battery impacts the design and form factor of the device.
Lithium-ion batteries have a high energy density, both gravimetric and volumetric. They can store a substantial amount of energy in a relatively small and light package, making them ideal for a wide range of applications.
Why Lithium-ion is a Bad Idea
Albeit market standard, Li-on batteries do have enough flaws to warrant the question, why Li-on is a bad idea? For the use-cases today, Li-on maybe good enough, but its inherent limitations and potential environmental concerns do not paint a positive picture in the longer run.
Structural Quirks
The architecture of lithium-ion batteries, as intricate as it is, presents a series of vulnerabilities. Heat sensitivity tops the list. These batteries are akin to divas—performing spectacularly under optimal conditions but flinching at temperature extremities. This sensitivity raises concerns about long-term stability and safety.
The internal structure, while compact, is complex. It involves layers of sensitive materials, separators, and electrolytes—all of which must maintain integrity under various conditions. Any compromise to this intricate arrangement not only affects performance but raises safety concerns, highlighted by instances of batteries overheating or even catching fire.
Environmental and Resource Concerns
Despite their ubiquity, lithium-ion batteries tote along an environmental baggage. The mining of lithium, an essential component, implicates a series of environmental issues including habitat destruction and soil contamination. As we extract this metal at an unprecedented pace to satiate our insatiable appetite for energy, the spectre of resource depletion looms large.
Cobalt, another critical component, is not only scarce but brings along a dark tale of unethical mining practices. The quest to extract this metal has often been tinted with reports of child labor and hazardous working conditions, painting a grim picture of the human cost of our energy needs.
The Degradation Dilemma
Each charge and discharge cycle chips away at the vitality of Li-on batteries, leading to a reduced energy storage capacity over time.
Effort’s to Save a Battery’s Life
So this brings us to the ‘what’s being done about it’ section of the article. And yes, a lot is being done. Techies are no dummies, they understand that solving the battery problem is key to solving many of the futuristic tech problems. After all, how can we insert chips into our brain and only to then go plug ourselves in for hours while they charge. Today’s batteries are in no way feasible for tomorrow’s technologies. I have summarised below, five solutions that the industry is currently trying to work on.
Energy Harvesting Technologies
Energy harvesting mechanisms are venturing beyond traditional paradigms, tapping into ambient energy sources with unprecedented efficiency. Thermoelectric generators, for instance, are being optimised to convert temperature gradients into electrical energy, with advancements in materials like bismuth telluride enhancing conversion efficiency. Piezoelectric materials are under the lens too, with innovations enabling the conversion of mechanical stresses, borne from actions like walking or ambient vibrations, into electrical energy.
Solid-State Batteries
The clamour around solid-state batteries stems from their elimination of liquid electrolytes, mitigating risks of leakage, and thermal runway. They employ solid electrolytes like lithium phosphorus oxynitride (LiPON) or sulfide-based ceramics that enhance ionic conductivity. With a higher energy density, these batteries promise extended lifespans and faster charging times, underpinned by improved thermal stability and safety.
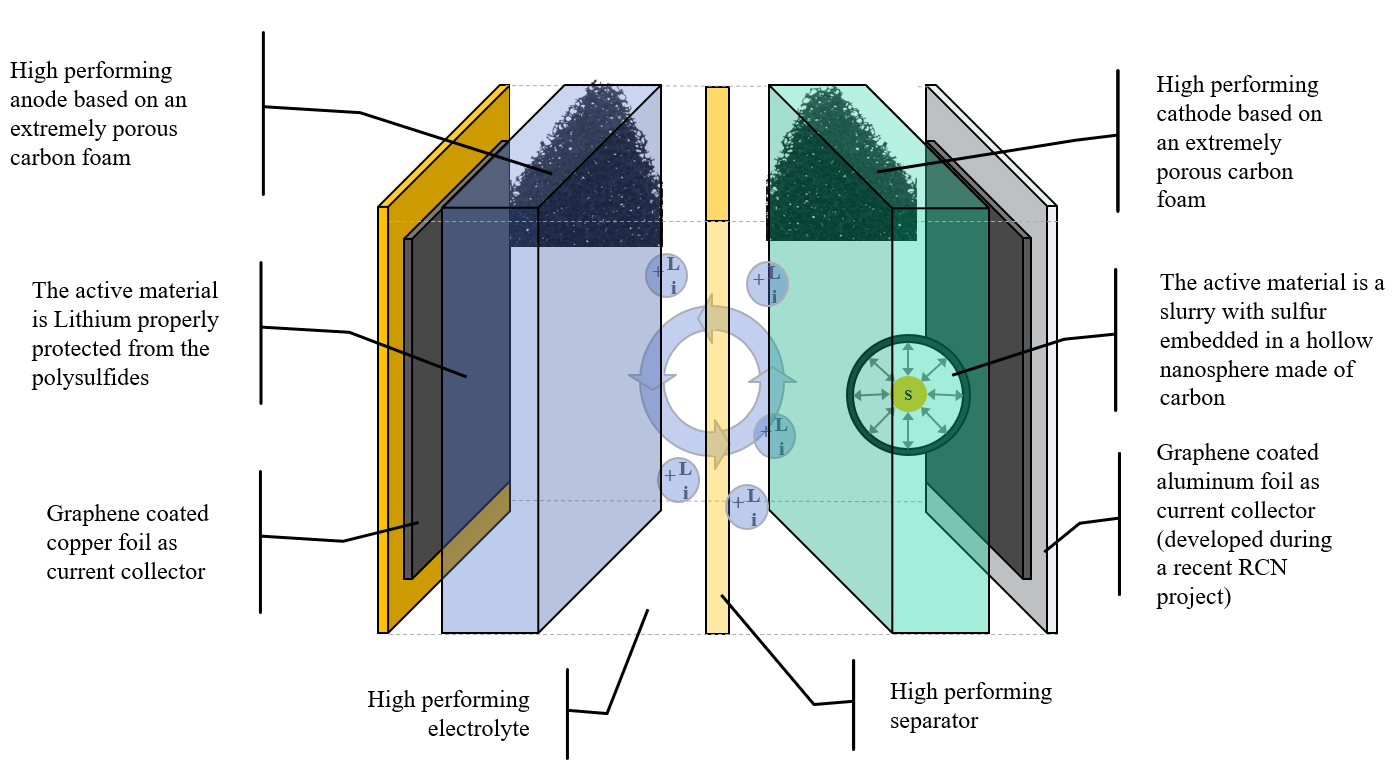
Graphene Batteries
Graphene batteries are a merger of high energy storage capacities and rapid energy release rates. Graphene, a single layer of carbon atoms arranged in a hexagonal lattice, boasts exceptional electrical conductivity, thermal stability, and mechanical strength. In battery technology, its incorporation augments electron mobility, enabling rapid charging and discharging while enhancing energy density, fostering batteries that are not just fast, but enduring.
AI and Machine Learning
AI is embedding intelligence into battery design and management. Algorithms are being trained to analyse complex patterns in battery usage and degradation, enabling predictive maintenance and optimised usage. Machine learning models, like Google’s DeepMind, are venturing into territory where they can predict battery lifespans with pinpoint accuracy, facilitating preemptive interventions and enhancing longevity.
Bio-Inspired Designs
In the biological realm, enzymes and proteins are the focal points. Bio-batteries that harness enzymes to catalyse reactions between fuels like glucose and ambient oxygen are under development, promising eco-friendly batteries with enhanced energy densities. Moreover, artificial photosynthesis systems are being honed to convert solar energy into chemical energy efficiently, mimicking the natural process to store energy in chemical bonds.
The Ideal Battery
In order to solve the battery problem, we don’t need to look out into the universe, we just need to look within. This isn’t some mindful woo woo stuff, I mean it for real. If you look within, you are going to see an exemplary piece of technology that functions on very little charge, is excellent at preserving energy and can function under high levels of stress with little to no time for recharge and rest. Sure it goes through entropy and decay just like everything else in the universe, but lithium-ion batteries are no match to us. We design AI by getting insights on how our brain functions. Similarly, we need to design batteries by drawing insights from how living forms on the planet function.
Attributes of an Ideal Battery
Here’s the five attributes that would characterise an ideal battery. This is obviously not an exhaustive list but I thought of pinning down on the 5 most crucial traits that an ideal battery must possess.
Efficiency: It should operate on lower charge, akin to the energy conservation mechanisms inherent in living organisms.
Longevity: The ability to run for extended periods, drawing inspiration from the enduring energy reserves of certain fauna and flora.
Self-Charging: A background recharging mechanism, reminiscent of how organisms continuously derive energy from their surroundings.
Durability: Crafted to withstand the tests of time, with a lifespan extending into centuries, a nod to the enduring nature of geological formations.
Sustainability: Eco-friendly and built with reusable materials, ensuring harmony with the environment and an escape from the constraints of finite resources.
Big Ask? Absolutely. But I wouldn’t go as far as to say it is unachievable. Think of the opportunities that having such a battery could achieve for the world. We could not only have cars but entire airlines and train systems functioning just on batteries. The positive impact on the environment is going to be BIG. Elon Musk can colonise Mars without worrying about the batteries running out. And also we will never have to worry about plugging our devices ever again! Just the thought of such a future makes me want to fast-forward my life to that.
Of course that would also mean that AI will have finally taken over the world with batteries that never die and humanity is under the occupation of an alien technology. I think I can live with that as long as I get to be a cyborg!
But on a serious note, as we stand on the brink of unprecedented technological evolution, the question isn’t if we can achieve this feat, but when. And more importantly, how can each one of us contribute to accelerating this journey?







This is an excellent article and I love the way it describes the life-cycle of a battery analogous to life-cycle of human beings. And of course, I wasn't aware about all the other potential replacement of these Lithium batteries.